All large-scale human genomic studies funded by NIH must register in dbGaP, an NIH repository for human genomic data, and NIH also encourages the registration of non-NIH funded studies in dbGaP. This page provides step-by-step instructions for registering a study in dbGaP.
Registering NIH-Funded Studies
This section describes how to register and submit data from NIH-funded studies to dbGaP.
- Submitting data to dbGaP is not a requirement under the GDS Policy, however, all human studies are registered in dbGaP even if they are not submitted to dbGaP.
- Some ICs or data sharing agreements require submission to specific repositories. However, the study must still be registered in dbGaP.
- Studies that have been granted an alternate data sharing plan may not have to submit data to dbGaP, but must still register the study in dbGaP.
| Identify appropriate Genomic Program Administrator (GPA) | |
|
|
Each funding institute or center (IC) has designated staff, referred to as GPAs, to assist investigators with registering and submitting genomic data. Note that an investigator cannot register a study in dbGaP by themselves.
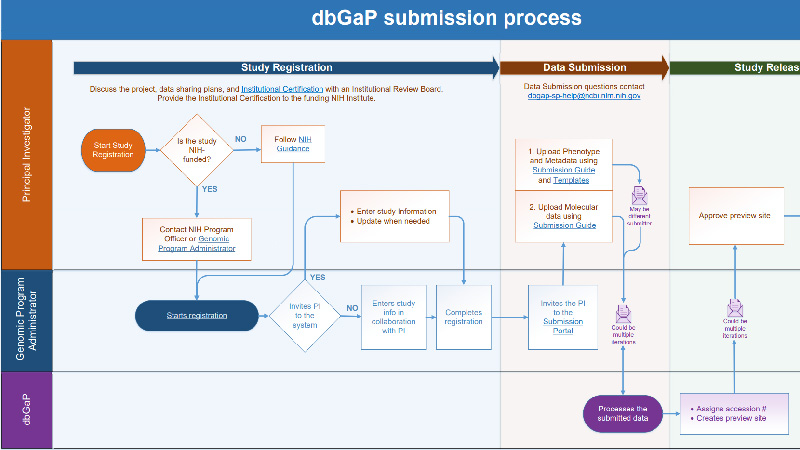
dbGaP Submission process Use this document to follow along with the dbGaP data submission process. |
| Send required documents and information to GPA | |
|
|
Prepare and obtain an institutional review board (IRB) review for a signed Institutional Certification, which will be required at Just in Time for NIH funded researchers, before submitting data.
Gather basic study information, which must be later
entered in dbGaP during registration. Below is a list of standard
information collected at this stage.
|
| GPA registers the study in dbGaP | |
|
|
The GPA will initiate registration in the dbGaP system using the Principal Investigator (PI) name, study name, and grant number (extramural) or protocol number (intramural) when the basic study information has been received and the Institutional Certifications have been approved. |
| (Optional) The Principal Investigator (PI) receives an “invite” from the dbGaP system | |
|
|
Once the GPA has initiated the registration in dbGaP, the PI may receive an email with instructions for completing the study registration process. |
| PI completes the study registration in dbGaP | |
|
|
The PI (or designee) enters basic study information in dbGaP (see Step 2 above), and can update as needed
Complete a Study Registration | 5 minThis video covers the steps that PI's perform to complete registration of their study. |
| GPA verifies the study registration information. | |
|
|
|
| PI submits data to dbGaP and/or another repository | |
|
|
Under the NIH GDS Policy, large-scale human genomic studies must be registered in dbGaP, but the data may be submitted to dbGaP and/or another repository, per the approved GDS plan. Step 7a: If submitting data to dbGaP
Step 7b: If submitting data to a repository other than dbGaP
|
Registering a Non-NIH Funded Study in dbGaP
| Pre-Submission: Identify the NIH Institute, Center, or Office that is most closely aligned with the purpose of the research to be submitted | |
|
|
The decision to accept non-NIH-funded study data is made by the NIH Institute, Center, or Office. Investigators should send the information described below to a Genomic Program Administrator (GPA) at the most relevant NIH Institute, Center, or Office (full list of ICOs) |
| Collect required documents and information and submit to GPA | |
|
|
The investigator should provide the following information to the GPA:
The SO is generally a senior official at the investigator’s institution who is credentialed through the NIH eRA Commons system and is authorized to enter the institution into a legally binding contract and sign on behalf of an investigator who has submitted data or a data access request to NIH. The institutional official is typically an academic administrator at the level of Vice President or above or a Dean. |
| The NIH GPA reviews the submission | |
|
|
Acceptance of the submission is based upon a consideration of the value of the data to the scientific community and the availability of NIH resources necessary to share the data. Scientific value can be assessed through considerations of IC priority, publication plans, and the quality and quantity of the data.
After review, the GPA will contact the investigator with any questions and/or to notify them of the IC’s decision, and also whether the option of donating funds as a conditional gift to support the deposition and storage of non-NIH-funded genomic research data in dbGaP is appropriate. |
| If accepted, begin registration process | |
|
|
If accepted, please follow the instructions starting at Step 3: GPA registers the study in dbGaP in How to Register and Submit a Study in dbGaP. |